
Watch the Bstent® Overview Video:
What is Bstent®?
Created by Dr. Bahman Guyuron, MD, FACS
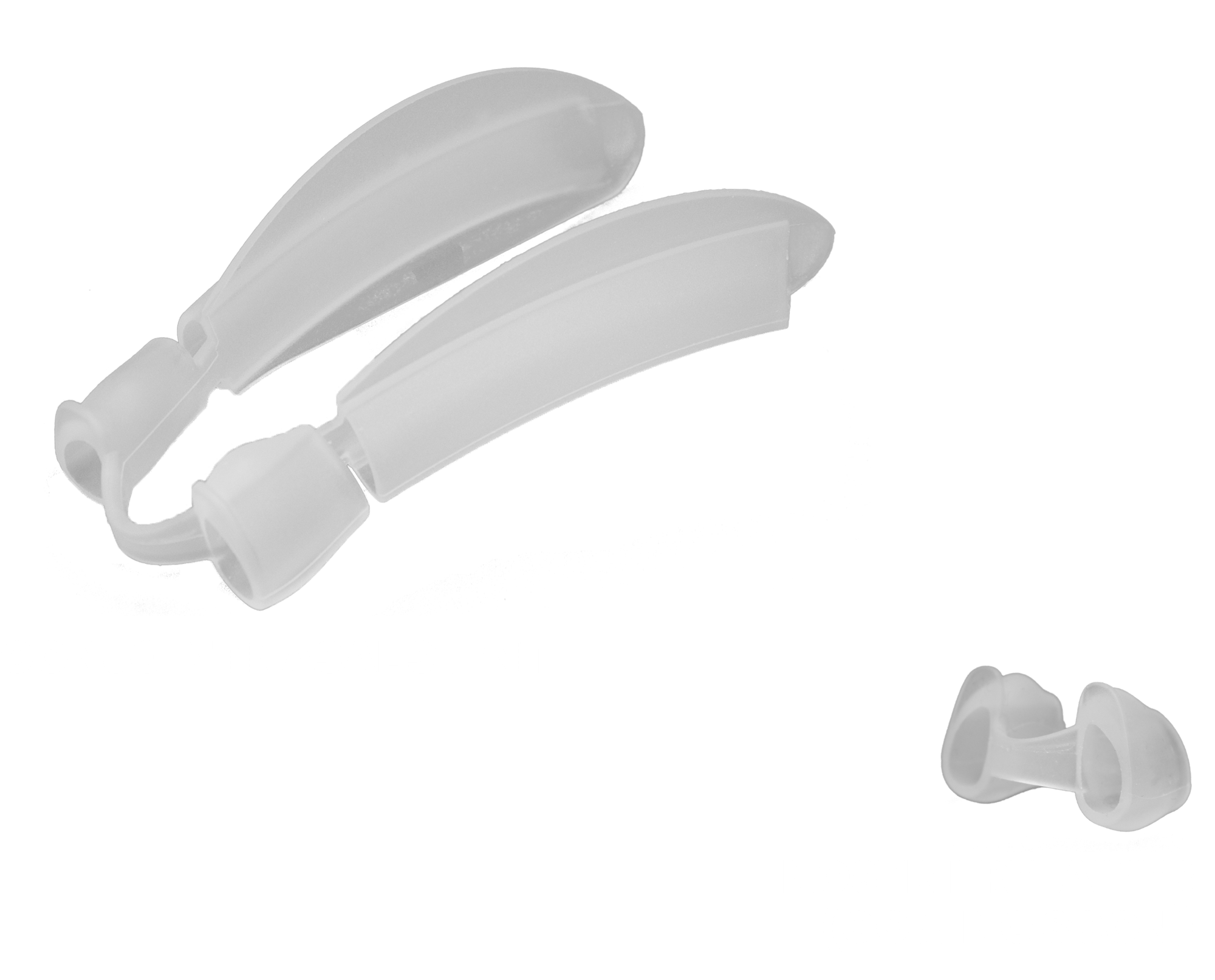
Created by Dr. Bahman Guyuron, MD, FACS, an internationally recognized educator and Innovator in the plastic surgery field, the patent pending Bstent® Conjoint Nasal Stent combines the features and benefits of nostril retainers and septal stents into a single, dual-purpose device, without the need for fixation. Bstent® can be used in two configurations, as directed by a physician: (1) as a Conjoint Nasal Stent (as originally packaged), or (2) as a Nostril Retainer, by removing both arms after the initial recovery period. Bstent® Nostril Retainers will be available soon for separate purchase.
Bstent® Features & Benefits
%20(2800%20x%203000%20px).png?width=2000&height=1410&name=Copy%20of%20Bstent%C2%AE%E2%80%99s%20Septal%20Splint%20is%20slightly%20shorter%20than%20alternative%20splints.%20(2000%20x%201080%20px)%20(2800%20x%203000%20px).png)
Bstent® vs Standard Splints
%20(2800%20x%203000%20px)%20copy.png?width=2000&height=659&name=Copy%20of%20Bstent%C2%AE%E2%80%99s%20Septal%20Splint%20is%20slightly%20shorter%20than%20alternative%20splints.%20(2000%20x%201080%20px)%20(2800%20x%203000%20px)%20copy.png)
Intended Use
Designed for use postoperatively
to provide septum support, prevent nostril collapse, and permit potential nasal breathing.
Indications for Use
Appropriate for use after Rhinoplasty, Turbinate reduction, Septoplasty, or Rhinoseptoplasty.